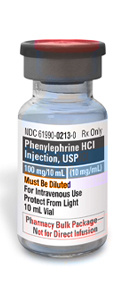
Phenylephrine Hydrochloride Injection, USP
Products > Phenylephrine Hydrochloride Injection, USP
Prescribing Information : Click here
Generic Name
Phenylephrine Hydrochloride Injection, USP
Reference Listed Drug
Vazculep®
NDC
61990-0213-1
Safety Data Sheet
Pack Size
1 Vial
Strength
10 mg/mL
Form
10 mL Vial
GTIN
00361990021315
Therapeutic Class
Vasopressors
Therapeutic Equivalence Rating
AP1
Container Closure is not made with natural rubber latex
Preservative Free
YES
Gluten Free
YES
ITEM CODES
Amerisource Bergen
10238277
Cardinal
5669460
McKesson
1566165
Morris & Dickson
198879
<p><strong>Phenylephrine Hydrochloride Injection for Intravenous Injection</strong></p>
<p> </p>
<p><strong>INDICATION AND IMPORTANT SAFETY INFORMATION</strong></p>
<p> </p>
<p><strong>INDICATIONS AND USAGE</strong></p>
<p>Phenylephrine hydrochloride injection is an alpha-1-adrenergic receptor agonist indicated for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia.</p>
<p> </p>
<p><strong>WARNINGS AND PRECAUTIONS</strong></p>
<p><strong>Exacerbation of Angina, Heart Failure, or Pulmonary Arterial Hypertension:</strong> Because of its increasing blood pressure effects, Phenylephrine hydrochloride injection can precipitate angina in patients with severe arteriosclerosis or history of angina, exacerbate underlying heart failure, and increase pulmonary arterial pressure.</p>
<p> </p>
<p><strong>Peripheral and Visceral Ischemia:</strong> Phenylephrine hydrochloride injection can cause severe excessive peripheral and visceral vasoconstriction and ischemia to vital organs, particularly in patients with extensive peripheral vascular disease.</p>
<p> </p>
<p><strong>Skin and Subcutaneous Necrosis</strong>: Extravasation of Phenylephrine hydrochloride injection can cause necrosis or sloughing of tissue. The infusion site should be checked for free flow. Care should be taken to avoid extravasation of Phenylephrine hydrochloride injection.</p>
<p> </p>
<p><strong>Bradycardia:</strong> Phenylephrine hydrochloride injection can cause severe bradycardia and decreased cardiac output.</p>
<p> </p>
<p><strong>Allergic Reactions:</strong> Phenylephrine hydrochloride injection contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.</p>
<p> </p>
<p><strong>Renal Toxicity:</strong> Phenylephrine hydrochloride injection can increase the need for renal replacement therapy in patients with septic shock. Monitor renal function.</p>
<p> </p>
<p><strong>Risk of Augmented Pressor Effect in Patients with Autonomic Dysfunction:</strong> The increasing blood pressure response to adrenergic drugs, including Phenylephrine hydrochloride injection, can be increased in patients with autonomic dysfunction, as may occur with spinal cord injuries.</p>
<p> </p>
<p><strong>Pressor Effect with Concomitant Oxytocic Drugs:</strong> Oxytocic drugs potentiate the increasing blood pressure effect of sympathomimetic pressor amines including Phenylephrine hydrochloride injection, with the potential for hemorrhagic stroke.</p>
<p> </p>
<p><strong>ADVERSE REACTIONS</strong></p>
<p>The most common adverse reactions during treatment are nausea, vomiting, and headache. Other reported adverse events include Cardiac Disorders: reflex bradycardia, lowered cardiac output, ischemia, hypertension, arrhythmias; Gastrointestinal Disorders: epigastric pain; Nervous System Disorders: blurred vision, neck pain, tremors; Vascular Disorders: hypertensive crisis; Respiratory, Thoracic and Mediastinal Disorders: Dyspnea; Skin and Subcutaneous Tissue Disorders: Pruritis.</p>
<p> </p>
<p><strong>DRUG INTERACTIONS</strong></p>
<p><strong>Agonistic effects (increase in Phenylephrine hydrochloride injection blood pressure effect):</strong> can occur with monoamine oxidase inhibitors (MAOI), oxytocin and oxytocic drugs, tricyclic antidepressants, angiotensin and aldosterone, atropine, steroids, norepinephrine transporter inhibitors, ergot alkaloids.</p>
<p> </p>
<p><strong>Antagonistic effect (decrease in Phenylephrine hydrochloride injection blood pressure effect):</strong> can occur with alpha-adrenergic antagonists, phosphodiesterase Type 5 inhibitors, mixed alpha- and beta- receptor antagonists, calcium channel blocker, benzodiazepines and ACE inhibitors, centrally acting sympatholytic agents.</p>
<p> </p>
<p><strong>IMPORTANT ADMINISTRATION INSTRUCTIONS</strong></p>
<p>Phenylephrine hydrochloride injection, 10mg/mL must be diluted before administration as an intravenous bolus or continuous intravenous infusion. Refer to Full Prescribing Information for dilution instructions. Do not use if the solution is colored or cloudy, or if it contains particulate matter. Diluted solution should not be held for more than 4 hours at room temperature or for more than 24 hours under refrigerated conditions. Dispensing from a pharmacy bulk vial should be completed within 4 hours after the vial is penetrated.</p>
<p> </p>
<p><strong>USE IN SPECIFIC POPULATIONS</strong></p>
<p><strong>Pregnancy</strong> <u>Risk Summary</u>: Data from randomized controlled trials and meta-analyses with phenylephrine hydrochloride injection use in pregnant women during Cesarean section have not established a drug-associated risk of major birth defects and miscarriage. There are no data on the use of phenylephrine during the first or second trimester.</p>
<p> </p>
<p><strong>Lactation</strong> <u>Risk Summary</u>: There are no data on the presence of phenylephrine hydrochloride injection or its metabolite in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Phenylephrine hydrochloride injection and any potential adverse effects on the breastfed infant from Phenylephrine hydrochloride injection or from the underlying maternal condition.</p>
<p> </p>
<p><strong>Pediatric Use</strong>: Safety and effectiveness in pediatric patients have not been established.</p>
<p> </p>
<p><strong>Geriatric Use:</strong> Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.</p>
<p> </p>
<p><strong>Hepatic Impairment:</strong> In patients with liver cirrhosis (Child Pugh Class B and Class C], dose-response data indicate decreased responsiveness to phenylephrine. Start dosing in the recommended dose range but consider that you may need to give more phenylephrine in this population.</p>
<p> </p>
<p><strong>Renal Impairment:</strong> In patients with end stage renal disease (ESRD), dose-response data indicate increased responsiveness to phenylephrine. Consider starting at the lower end of the recommended dose range, and adjusting the dose based on the target blood pressure goal.</p>
<p> </p>
<p><strong>OVERDOSAGE</strong></p>
<p>Overdose of Phenylephrine hydrochloride injection can cause rapid rise in blood pressure. Symptoms of overdose include headache, vomiting, hypertension, reflex bradycardia, a sensation of fullness in the head, tingling of the extremities, and cardiac arrhythmias including ventricular extrasystoles and ventricular tachycardia.</p>
<p> </p>
<p><strong>For additional Safety Information, please see Full Prescribing Information.</strong></p>
<p> </p>
<p><strong>You are encouraged to report adverse drug events to Provepharm at 1-833-727-6556 or to the FDA by visiting www.fda.gov/medwatch or by calling 1-800-FDA-1088.</strong></p>
