BLUDIGO™ (Indigotindisulfonate Sodium Injection)
-
Reference Listed Drug:
BLUDIGO™
-
Form:
Ampule
-
Strength:
5 mL
-
Pack Size:
5 Ampules
-
NDC:
81284-315-05
-
Safety Data Sheet:
Click Here PDF
Disclaimer:
<p><span style="color:#c0392b;">Sold by Sagent Pharmaceuticals, effective December 1, 2025</span></p>
Physostigmine Salicylate Injection
-
Form:
Injection for IV
-
Strength:
2 mg/5 mL (0.4 mg/mL) Injection for Intravenous Use
-
Pack Size:
5 x 5 mL Ampules
-
NDC:
81284-831-05
-
Safety Data Sheet:
Click Here PDF
Disclaimer:
<p><strong>Physostigmine Salicylate Injection has not been found by FDA to be safe and effective, and its labeling has not been approved by FDA. For further information about unapproved drugs, <a href="https://www.fda.gov/drugs/enforcement-activities-fda/unapproved-drugs">click here.</a></strong></p> <p><br /> <strong>Disclaimer: Anticholium® (physostigmine salicylate) is not FDA approved, but is imported with foreign, non-US labeling under a temporary importation license to address supply shortage. <a href="https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b90b50e8-c8f1-4f2d-be6e-4d269b8c362c">For more information click here.</a></strong></p>
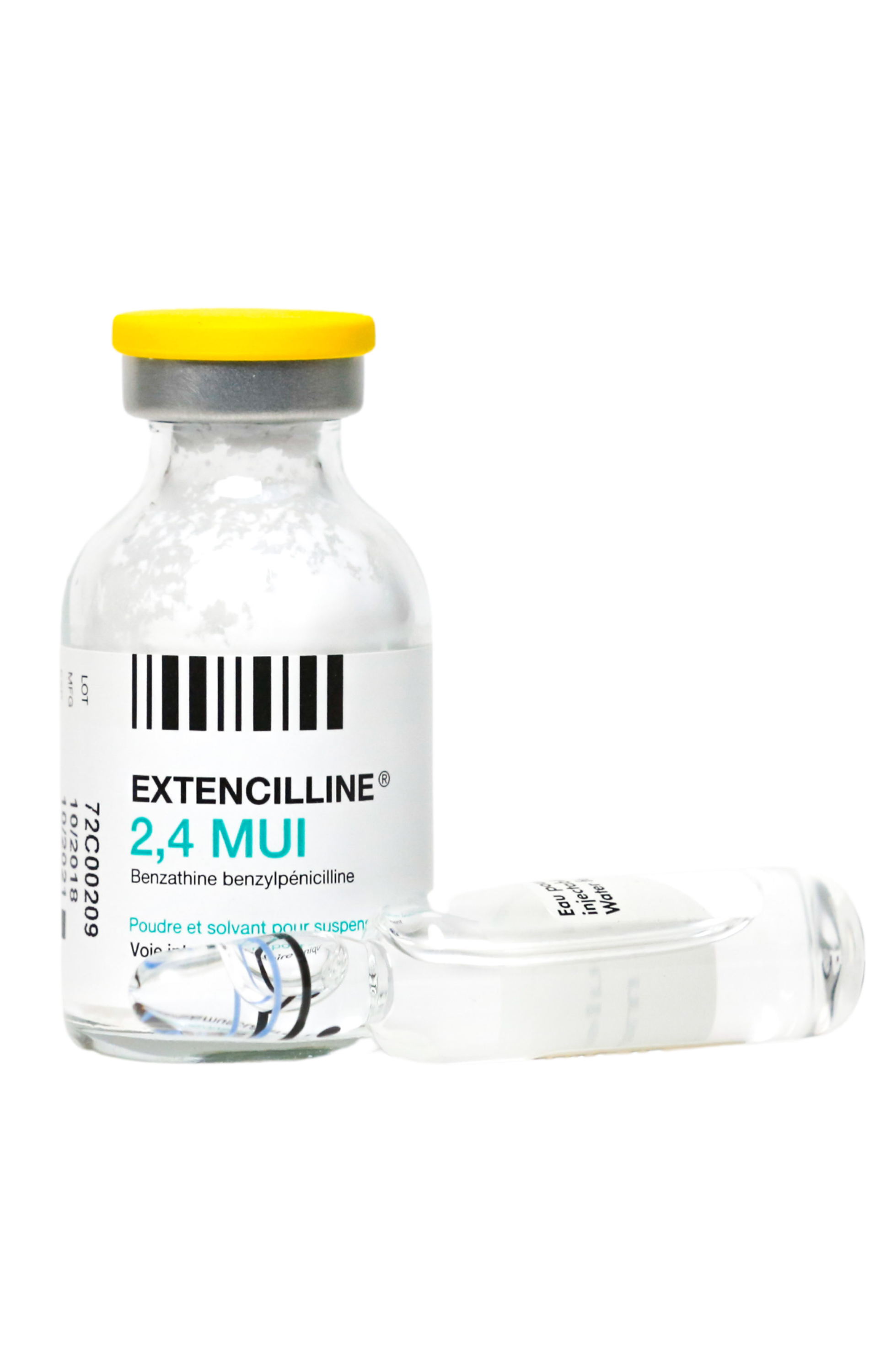
Extencilline (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection
-
Form:
Powder and solvent for suspension for injection
-
Strength:
1,200,000 units
-
Pack Size:
20 mL vial of powder for suspension + 5 mL of water for injection in ampule
-
NDC:
81284-521-01
-
Safety Data Sheet:
Click Here PDF
Disclaimer:
<p><strong>Due to the ongoing shortages of Bicillin® L-A (penicillin G benzathine injectable suspension) in the United States, Laboratoires Delbert in conjunction with Provepharm Inc. (Provepharm) and Direct Success, Inc. (Direct Success) is coordinating with the U.S. Food and Drug Administration (FDA) to temporarily import Extencilline, (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection, 1,200,000 units and 2,400,000 units into the U.S. market. Delbert’s Extencilline, (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection, 1,200,000 units and 2,400,000 units marketed in France and manufactured in Italy for Delbert, is not FDA-approved. Benzathine benzylpenicillin is another name for Penicillin G benzathine. <a href="https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a026d0c-6f91-4ee3-b193-b2186a37e7ca">For more information, click here.</a></strong></p>
-
Form:
Powder and solvent for suspension for injection
-
Strength:
2,400,000 units
-
Pack Size:
20 mL vial of powder for suspension + 5 mL of water for injection in ampule
-
NDC:
81284-522-01
-
Safety Data Sheet:
Click Here PDF
Disclaimer:
<p><strong>Due to the ongoing shortages of Bicillin® L-A (penicillin G benzathine injectable suspension) in the United States, Laboratoires Delbert in conjunction with Provepharm Inc. (Provepharm) and Direct Success, Inc. (Direct Success) is coordinating with the U.S. Food and Drug Administration (FDA) to temporarily import Extencilline, (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection, 1,200,000 units and 2,400,000 units into the U.S. market. Delbert’s Extencilline, (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection, 1,200,000 units and 2,400,000 units marketed in France and manufactured in Italy for Delbert, is not FDA-approved. Benzathine benzylpenicillin is another name for Penicillin G benzathine. <a href="https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a026d0c-6f91-4ee3-b193-b2186a37e7ca">For more information, click here.</a></strong></p>