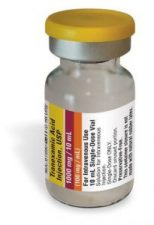
Tranexamic Acid Injection, USP
Products > Tranexamic Acid Injection, USP
Prescribing Information : Click here
Generic Name
Tranexamic Acid Injection, USP
Reference Listed Drug
Cyklokapron®
NDC
61990-0611-2
Safety Data Sheet
Pack Size
10 Vials
Strength
100 mg/mL
Form
10 mL Vial
GTIN
00361990061120
Therapeutic Class
Hemostatic
Therapeutic Equivalence Rating
AP
Container Closure is not made with natural rubber latex
Preservative Free
YES
Gluten Free
YES
Closure Size
20 mm
Container Size
10 mL
ITEM CODES
Amerisource Bergen
10230101
Cardinal
5568068
McKesson
3986874
Morris & Dickson
898668
<h3><strong>Tranexamic Acid Injection for Intravenous Injection</strong></h3>
<p> </p>
<p><strong>INDICATION AND IMPORTANT SAFETY INFORMATION</strong></p>
<p><strong>INDICATIONS AND USAGE</strong></p>
<p>Tranexamic acid injection is an antifibrinolytic indicated in patients with hemophilia for short-term use (2 to 8 days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction.</p>
<p> </p>
<p><strong>CONTRAINDICATIONS</strong></p>
<p>Tranexamic acid injection is contraindicated in:</p>
<ul class="normal">
<li>
<p>Patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by tranexamic acid injection in such patients.</p>
</li>
<li>
<p>Patients with active intravascular clotting.</p>
</li>
<li>
<p>Patients with hypersensitivity to tranexamic acid or any of the ingredients.</p>
</li>
</ul>
<p> </p>
<p><strong>WARNINGS AND PRECAUTIONS</strong></p>
<p> </p>
<p><strong>Thromboembolic Risk:</strong> Tranexamic acid injection is contraindicated in patients with active intravascular clotting. Risk of Thrombosis is increased with concomitant use of products that are pro-thrombotic, including Factor IX, anti-inhibitor coagulant concentrates, and hormonal contraceptives: Avoid concomitant use.</p>
<p> </p>
<p><strong>Seizures:</strong> Tranexamic acid may cause seizures, including focal and generalized seizures. Inadvertent injection into the neuraxial system may result in seizures. Tranexamic acid is not approved and not recommended for neuraxial administration. Discontinue tranexamic acid if seizures occur.</p>
<p> </p>
<p><strong>Hypersensitivity Reactions:</strong> Cases of hypersensitivity reactions, including anaphylactic reactions have occurred. Tranexamic acid is contraindicated in patients with a history of hypersensitivity to tranexamic acid. Discontinue treatment with tranexamic acid if a serious reaction occurs, provide appropriate medical management, and do not restart treatment.</p>
<p> </p>
<p><strong>Visual Disturbances:</strong> Visual or ocular adverse effects may occur. Discontinue use if visual or ocular symptoms occur. For patients expected to be treated for greater than 3 months, consider ophthalmologic monitoring.</p>
<p> </p>
<p><strong>Dizziness:</strong> Tranexamic acid may cause dizziness. Concomitant use of other drugs that may also cause dizziness may worsen this effect. Advise patients to avoid driving or using machines until they know how tranexamic acid affects them.</p>
<p> </p>
<p><strong>ADVERSE REACTIONS</strong></p>
<p>The most commonly occurring adverse reactions are nausea, vomiting, diarrhea, allergic dermatitis, giddiness, hypotension, and thromboembolic events. Anaphylaxis or anaphylactoid reactions have been reported that are suggestive of a causal relationship.</p>
<p> </p>
<p><strong>DRUG INTERACTIONS</strong></p>
<p>Prothrombotic Medical Products: Avoid concomitant use; can further increase the risk of thromboembolic adverse reactions associated with tranexamic acid.</p>
<p> </p>
<p><strong>IMPORTANT ADMINISTRATION INSTRUCTIONS</strong></p>
<p>Tranexamic acid injection should NOT be mixed with blood. The drug is a synthetic amino acid and should NOT be mixed with solutions containing penicillin. Discard any unused portion.</p>
<p> </p>
<p><strong>USE IN SPECIFIC POPULATIONS</strong></p>
<p> </p>
<p><strong>Pregnancy</strong> <u>Risk Summary</u>: Tranexamic acid is known to pass through the placenta and appears in cord blood at concentrations approximately equal to maternal concentration. It is not known whether tranexamic acid use in pregnant women may cause a drug-associated risk of miscarriage or adverse maternal or fetal outcomes.</p>
<p> </p>
<p><strong>Lactation</strong> <u>Risk Summary</u>: Published literature reports the presence of tranexamic acid in human milk. There are no data on the effects of tranexamic acid on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for tranexamic acid injection and any potential adverse effects on the breastfed child from tranexamic acid injection or from the underlying maternal condition.</p>
<p> </p>
<p><strong>Females and Males of Reproductive Potential</strong> <u>Contraception</u>: Concomitant use of tranexamic acid injection with hormonal contraceptives may increase the risk for thromboembolic adverse reactions. Avoid concomitant use of tranexamic acid with hormonal contraceptives.</p>
<p> </p>
<p><strong>Pediatric Use</strong>: There are limited data concerning the use of tranexamic acid in pediatric patients with hemophilia who are undergoing tooth extraction. The limited data suggest that there are no significant pharmacokinetic differences between adults and pediatric patients.</p>
<p> </p>
<p><strong>Geriatric Use:</strong> Care should be taken in dose selection, and it may be useful to monitor renal function – in the event of renal impairment refer to the Renal Impairment instructions below and in the full prescribing information.</p>
<p> </p>
<p><strong>Renal Impairment:</strong> This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Reduce the dosage of tranexamic acid injection in patients with renal impairment, based on the patient’s serum creatinine. Refer to the full prescribing information for recommended dosage instructions.</p>
<p> </p>
<p><strong>OVERDOSAGE</strong></p>
<p>Cases of overdosage of tranexamic acid injection have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, hypotensive, thromboembolic, neurologic, myoclonus and rash.</p>
<p> </p>
<p><strong>For additional Safety Information, please see Full Prescribing Information.</strong></p>
<p><strong>You are encouraged to report adverse drug events to Provepharm at 1-833-727-6556 or to the FDA by visiting www.fda.gov/medwatch or by calling 1-800-FDA-1088.</strong></p>
